Engage students with a simple demonstration of ice melting. Show them a piece of ice and ask what they think will happen if it is left in the sun. This will introduce the concept of melting and set the stage for exploring the changing states of matter.
Go to the LessonLearning Objectives
- Understand that ice is water in a solid state and melts into liquid when heated.
- Learn the process of evaporation where water changes from a liquid to a gas when heated.
- Recognize that condensation is the process where water vapor cools and changes back into a liquid.
- Identify freezing as the process where liquid water changes into a solid when cooled.
- Understand that heating or cooling water can change its state from solid to liquid to gas and vice versa.
Introduction and Hook
Direct Instruction
Explain the concept of states of matter using water as an example. Discuss how ice, water, and steam represent solid, liquid, and gas states respectively.
Guided Exploration
Facilitate a discussion on how heating and cooling affect the state of water. Use examples like boiling water for steam and freezing water for ice.
Hands-On Activity
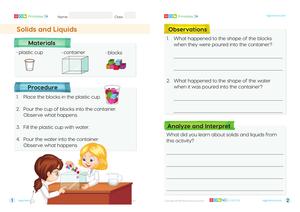
Conduct an experiment where students observe ice melting and water evaporating. Have them record their observations and discuss the changes they see.
Independent Practice
Check for Understanding
Review and Reflection
Have students reflect on what they learned about the changing states of matter. Encourage them to share examples from their daily lives where they observe these changes.
Assessment and Extension
Administer the 'The Changing States of Water' assessment to test comprehension of freezing, melting, and evaporation. This worksheet includes fill-in-the-blank and scenario-based questions.
Try the Quiz