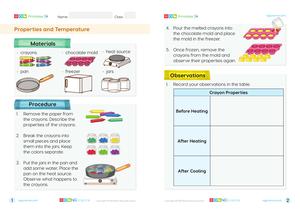
Begin the lesson by discussing the different states of water: solid, liquid, and gas. Use real-life examples like ice, water, and steam to engage students.
Go to the LessonLearning Objectives
- Understand the transformation of water from solid to liquid through the process of melting.
- Explain how heating liquid water leads to evaporation into water vapor.
- Describe the process of condensation, where water vapor cools and changes back into liquid water.
- Identify freezing as the process in which liquid water becomes solid ice when cooled.
- Recognize that the changing states of water are reversible processes.
Introduction and Hook
Direct Instruction
Explain the process of melting, evaporation, condensation, and freezing using the lesson content. Highlight the reversible nature of these changes.
Guided Exploration
Hands-On Activity
Conduct an experiment where students observe the melting of ice and the evaporation of water. Encourage them to record their observations and discuss the changes they observe.
Independent Practice
Check for Understanding
Ask students to explain the process of condensation and how it relates to the water cycle. Use their responses to gauge understanding.
Review and Reflection
Review the key concepts of the lesson by discussing the reversible nature of water's state changes. Encourage students to share what they found most interesting.
Assessment and Extension
Administer the 'Properties and Temperature' assessment to evaluate students' understanding of how temperature affects the states of matter.
Encourage students to take the unit quiz to test their comprehension of the lesson material.
Try the Quiz